Analysis from the first 24-months9 showed favourable effects on some secondary outcomes (e.g., CDR-SB and hippocampal atrophy) but not on the primary NTB endpoint. As some effects were observed at 24 months but cognitive decline was less than anticipated in the control group, it was proposed that the longer intervention may lead to more pronounced effects. A post-hoc analysis was performed using the Alzheimer’s Disease Composite Score (ADCOMS), which identified the cognitive and functional benefits of the specific multi-nutrient intervention, with 36% less decline in the active group16. The most recent analysis on this data applied a Time Component Test (TCT) to the primary NTB endpoint, the CDR-SB and hippocampal volume, and combined the outcomes to show 9 months of time savings relative to placebo over 24 months of treatment (9.0, 10.5, and 7.2 months for NTB, CDR-SB, and hippocampal volume respectively)17.
Results from 36 months of the intervention were published in 2021 showing significant positive benefits on cognition, function, and rates of brain atrophy (brain shrinkage) in the active group (Souvenaid) specifically.
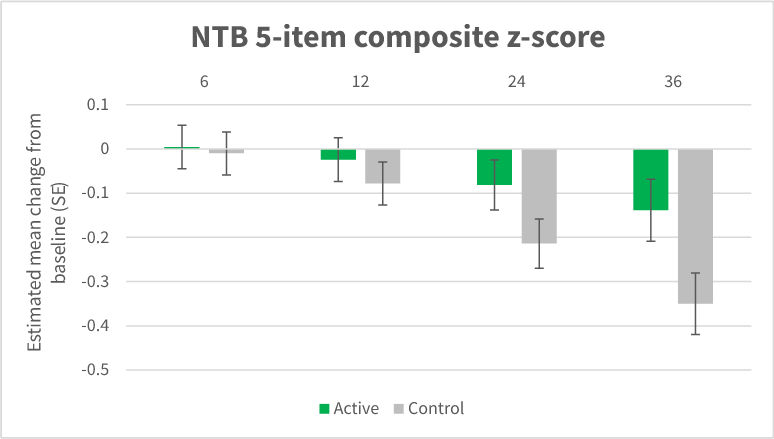
- Significant reductions in decline on the primary outcome of NTB measuring cognition (60% reduction in decline) as illustrated in figure 1.
- Statistically significant differences between groups in favour of the active group were observed for the CDR-SB (45% less worsening;), memory (76% less worsening;). Such a sustained benefit over 3 years has not been reported before in early AD. The CDR-SB benefits occurred in conjunction with several NTB items supporting the positive impact of the active intervention on cognition and function.
- The rates of deterioration for hippocampal, whole brain and ventricular volume (brain atrophy) were significantly less in the active than the control group, showing potential effect on disease pathology. The effect on hippocampal atrophy may be the basis for the memory benefit reported for the active group. The decline rates in hippocampal atrophy in the control group were typical of those for MCI/mild AD reported.
- A small to medium Cohen’s d effect size (0.25–0.31) similar to that of established clinically relevant AD treatment was found.
Subsequent post-hoc ADCOMS analysis showed a statistically significant slowing in change from baseline scores over 36 months, with 36% less worsening of disease in the active group compared with the control group. The TCT analyses on the 36-month results of the intervention are currently being prepared.
The active product was found to be safe, very well tolerated, and compliance with the supplement at 36 months was very high (means of 91.4% in the active group and 90.8% in the control group).
Given the observed positive impact on cognition, brain function and atrophy, the study demonstrated that long-term nutritional intervention with Souvenaid has the potential to alter disease trajectories over 3 years. The authors suggest that the duration of the nutritional intervention and early initiation in the disease may be factors that contribute to the observed benefits.